TMS Safety?
Not So Fast, Side Effects Could Last
What Is TMS?
Most people associate brain stimulation treatment with electroconvulsive therapy (ECT), or “shock therapy”.1 ECT, which involves a brief electrical stimulation on a patient’s brain while they are under anesthesia, has been used heavily for decades. It has been considered the gold standard for treating depression which has not responded adequately to the first line of treatment.1
While ECT is still thought of as a potent and effective treatment that is widely used across the country, it takes a toll on the patient’s memory and cognition which is part of the reason why transcranial magnetic stimulation or TMS has risen to prominence as a viable alternative treatment option.1
TMS uses rapidly alternating magnetic fields to stimulate specific areas of the brain.2 This form of therapy was first introduced in 1985 but it was not approved by the US Food and Drug Administration (FDA) until 2008.3
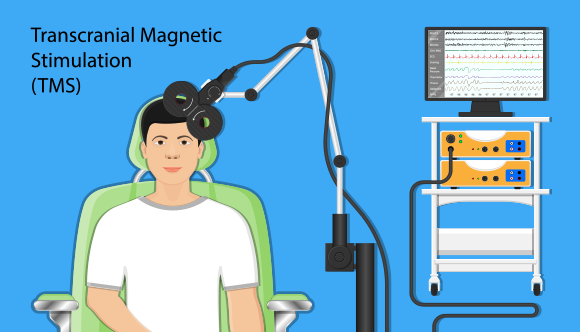
How Does TMS Work?
The patient remains awake and seated throughout the process. After the patient has removed any magnetic-sensitive objects, they are given earplugs to wear during treatment.4 A device is then attached to or held up to the patients head and electromagnetic energy is released with a loud repetitive clacking noise.
According to the American Psychiatry Association, TMS only has mild side effects including headaches, muscle twitches and localized pain at the stimulation site.2 Therapy frequency is determined based on individual response.4
How Effective Is TMS for the Treatment of Depression?
According to the University of North Carolina School of Medicine, TMS may not work for everyone. Not all patients respond to this therapy and their symptoms could worsen or intensify.5
Depression or major depressive disorder has a staggering prevalence of nearly 7 percent in the general population.3
For many patients affected with the condition, treatment options include a combination of medications and therapy. Selective Serotonin Reuptake Inhibitors are usually the first line of defense and the most commonly prescribed medications for patients dealing with depression.7 But, an estimated 30 percent of patients struggling with depression do not respond to this modality of treatment.3 If a person has not responded to the first medication they were prescribed, they are less and less likely to respond well to other medications available to them.3
When these options fail, doctors may recommend TMS as a course of treatment. However, TMS is not guaranteed to be effective and patients may not respond to this modality of treatment regardless of the number and frequency of sessions.5
If TMS is effective, an ongoing maintenance regimen is necessary to maintain positive treatment outcomes.5 This means that even if patients respond well to TMS, they may still experience a deep crash where intense symptoms can suddenly reappear once they stop treatment.
TMS is not guaranteed to be effective and patients may not respond to this modality of treatment regardless of the number and frequency of sessions.5
As for the efficacy of TMS in depression, the evidence is promising but not conclusive.3 In terms of treatment of obsessive compulsive disorder, posttraumatic disorder, or schizophrenia, TMS has not been shown to be effective.3
But according to most TMS treatment providers, the success rate of TMS is 70% or 80%, and for 50% of those patients, symptoms of depression disappear altogether after a single course of treatment.6 Some even claim they have achieved a success rate of 92.5%.6
But is there a catch? What are they not telling us?
Is TMS Non-invasive?
TMS and transcranial current stimulation (tCS), like all forms of brain stimulation, might be used to enhance brain function or disrupt activity.7 Both techniques are referred to and marketed as non-invasive brain stimulation.7
However, some researchers consider the term non-invasive in the context of TMS and transcranial current stimulation problematic:
“We argue that this term [non-invasive brain stimulation] is inappropriate and perhaps oxymoronic, as it obscures both the possibility of side-effects from the stimulation, and the longer-term effects (both adverse and desirable) that may result from brain stimulation.”7
Another major concern is that “the established tendency for the effects of TMS and tCS to spread from the target brain area to neighboring areas is in itself contrary to the definition of non-invasiveness.”7
While these targeted transcutaneous interventions may not be as invasive as physical insertion or incision, they do not meet the criteria for non-invasiveness either.7
The takeaway is that referring to TMS as a non-invasive technique is misleading because patients may interpret it to mean that its effects will necessarily be mild.7
What our research discovered
This is another aspect we investigated in our research, which included 22 dedicated TMS web pages on websites of 20 TMS treatment providers. This is the full list of TMS treatment providers whose websites we analyzed:
- Mayo Clinic
- Cleveland Clinic
- UCLA Medical Center
- Johns Hopkins Medicine
- McLean Hospital
- Massachusetts General Hospital
- UCSF Medical Center
- New York-Presbyterian Hospital-Columbia and Cornell
- Rush University Medical Center
- Cedars-Sinai Medical Center
- NYU Langone Hospitals
- Northwestern Memorial Hospital
We specifically looked for the phrase “non-invasive/noninvasive” and wanted to see how many TMS providers’ websites explicitly refer to TMS as non-invasive in the web copy. What we discovered was that the majority clearly labels TMS as non-invasive (14 out of 22 analyzed).
Is TMS Non-Invasive?
The chart shows the ratio of TMS providers’ web pages that claim that TMS is non-invasive vs. those that do not make such a claim. The total number of web pages analyzed was 22.

The majority of web pages analyzed (14 out of 22) clearly labels TMS as non-invasive in the web copy.
Here’s the list of hospitals whose dedicated TMS pages explicitly state that TMS is non-invasive:
- Mayo Clinic8
- Johns Hopkins Medicine9
- McLean Hospital10
- Massachusetts General Hospital11
- UCSF Medical Center12
- New York-Presbyterian Hospital-Columbia and Cornell13
- Rush University Medical Center14
- University of Michigan Hospitals-Michigan Medicine15
- Stanford Health Care-Stanford Hospital16
- Hospitals of the University of Pennsylvania-Penn Presbyterian17
- Brigham and Women’s Hospital18
- Houston Methodist Hospital19
What do TMS Providers Say about Side Effects?
Although this therapy is not new, there is still much that we do not know about it, which means the outcomes may not be predictable.7 In our analysis of the information presented on 20 TMS providers’ websites, we looked for the following:
- How many side effects were mentioned in the text,
- Which side effects were mentioned,
- Whether the text stated there were other side effects, but didn’t specify which.
On all the websites analyzed, there were 12 side effects mentioned in total:
- Headache
- Scalp discomfort at the site of stimulation/painful scalp sensations
- Tingling, spasms or twitching of facial muscles
- Lightheadedness
- Seizures
- Mania (particularly in people with bipolar disorder)
- Hearing loss (if there is inadequate ear protection during treatment)
- Fatigue
- Mild discomfort
- Toothache in some patients
How Many Side Effects Do TMS Providers Disclose?
The analysis that included 22 web pages discovered that most web pages mention NO side effects.

Furthermore, only Mayo Clinic’s dedicated TMS page implies that there may be other side effects by saying ”more study is needed to determine whether rTMS may have any long-term side effects,” which is still not explicitly saying that there may be other side effects besides the ones they listed.8 Other websites don’t even imply that their lists of side effects aren’t definitive.9-19
Vaguely about TMS
An important aspect of our web pages’ analysis is how vague language is used. Here we’re using the term “vague language” to denote those pieces of information that may be misleading to the reader. While we admit that our analysis may be subjective in part, it’s worth noting that patients suffering from depression and seeking a new form of treatment because all else has failed – are bound to be more impressionable and overlook the red flags.
Our analysis discovered that half the web pages analyzed contain elements of vague language. Here are some notable examples copied from analyzed web pages verbatim, with words and phrases in italics for emphasis:8-19
Side effects are generally mild to moderate and improve shortly after an individual session and decrease over time with additional sessions.
Generally, rTMS is considered safe and well-tolerated. However, it can cause some side effects.
The most common side effects include scalp discomfort and headaches, but these usually improve shortly after the treatment session is finished.
The examples above are considered vague because they don’t explicitly state the duration of the side effects, and use indefinite determiners like “some”. Basically, the above sentences don’t convey credible information to potential patients, and sound more like marketing copy.
Here are some more examples:8-19
-
Thousands of patients with depression in dozens of studies have shown that rTMS is safe and effective.
– Without a specific number, it’s an exaggeration
-
rTMS has been shown to be a safe and well-tolerated procedure that can be an effective treatment for patients with depression.
– Passive voice, doesn’t say by whom
-
Since no medications are administered, there are no cognitive or systemic after-effects, allowing patients to immediately return to regular activity.
– Jumping to conclusion – the fact that no medications are administered doesn’t mean there are no cognitive or systemic after-effects
-
Rush has performed more transcranial magnetic stimulation (TMS therapy) treatments than any other health system in the Chicago area.
– Logical fallacy – just because they performed the most of these, doesn’t mean it’s a safe procedure
-
In fact, recent studies have shown that TMS is comparable to some antidepressant medications in terms of seizure risk.
– There is no link to the studies
What Side Effects Do Patients Report?
The absence of systemic side effects may be what’s most concerning about TMS.20 According to Hall, the driving force behind Victims of TMS Action Group (VTAG), a Facebook community of patients who have experienced TMS side effects they had not been warned against, the list of potential TMS side effects is long, but the most alarming ones patients report include:
The Most Severe Side Effects
The list was compiled based on the interview with James Hall, and his activism in the Victims of TMS Action Group (VTAG) community.
- Severe anxiety/depression
- Severe fatigue
- Cognitive impairment/dysfunction
- Facial trauma
- Vision and hearing damage
- Heart failure
- Respiratory distress
- Seizures
- Epilepsy
- Psychosis
- Suicidal ideation
When asked what prompted him to effectively act as a spokesperson for TMS patients, James Hall relayed his personal story:
I was previously a reporting and analytics consultant at a fortune 10 bank. After years of dealing with poor treatment at work, I developed depression and anxiety. After a poor experience with medications, I swore them off. One day when visiting my GP, he recommended TMS as an alternative form of treatment. I was misled into thinking it was a non-invasive, risk-free treatment.
It shook me to the core. Not only did I lose my job after having spent 15 years building a career at the same company, but also my health. After living a corporate life for a very long time, I understood very well how horrible and inhuman large companies can be in treating their employees, and how they ruthlessly execute their business for the sake of profit. I learned over the years how they hide the truth by manipulating reporting requirements and massaging data. I had an epiphany and realized that TMS was a similar sham and all the propaganda used for marketing had leveraged the same tactics the bank I had worked at did. I recognized the same pattern of blatant disregard for people’s wellbeing and the right to know the truth. This motivated me beyond all reason to find out the truth behind TMS, to help others by treating them like human beings and exposing everything I have found.
Members of VTAG report that symptoms acquired from TMS injury linger for a long time. Hall says:
Still, Hall insists that:
Hall is of the opinion that all these symptoms cluster and contribute to each other, reinforcing the adverse effects on the body and mind:
Common vs Less Common Side Effects Reported by VTAG Community
The common symptoms reported by patients who have undergone TMS include:21
- Significantly worsening depression and anxiety (may also be newly “treatment-resistant”)
- Cognitive impairment such as short-term memory or functional memory loss and decreased ability to multitask
- Irritability
- Fatigue
- Panic attacks
- Increased suicidal ideation
- Chronic headaches
- Loss of balance
- Dizziness
- Tinnitus
The less common symptoms reported by patients who have undergone TMS include:21
- Hearing loss
- Eye injury
- Migraines
- Different forms of tachycardia
- Seizures and epilepsy
- Blood pressure problems
- Speech problems
- Muscle pain/weakness/twitching/cramping/tightness
- Insomnia
- Dissociation
- Environmental sensitivities to temperature, light, smell, and sound
Side effects depend on whether single-pulse TMS, paired-pulse TMS, low frequency rTMS, high frequency rTMS, or theta burst is used to administer therapy.22 Supporting these claims are publicly accessible records of adverse events reported to the FDA.23
Hall highlights the alarming fact that the only side effects disclosed by TMS clinics are the remote possibility of seizure, headaches and site discomfort. The actual consent form may only inform patients of possible headaches. None of the other side effects are disclosed by TMS clinics, although publicly available literature on TMS may list many more side effects and these clinics all have common complaints of neurological problems during treatment.
Hall goes on to say that the neurological harms present the same regardless of the previous psychological state of the patient.
Hall goes on to say that the neurological harms present the same regardless of the previous psychological state of the patient. TMS harms present very clearly as neurological harms, typically consistent with Post Concussive syndrome from electrical injury, which do not vary regardless of the diagnosis for which the patient was receiving TMS. While it may sometimes seem that there is a connection, e.g. a patient with anxiety experiences extreme worsening anxiety and panic as a result of TMS, many patients being treated for depression also experience severe anxiety and panic that they may have never experienced before, as a result of TMS.
When asked about the duration and severity of side effects, Hall says there is a loose positive association with increased frequency and duration. Generally speaking, people who have undergone TMS for a longer duration at a higher frequency/intensity have worse symptoms that last longer. That being said, the connection is loose. There are patients who have severe long-lasting side effects from just a single session of TMS.
According to Hall, the vast majority of TMS patients either have no improvement of symptoms and side effects or their symptoms worsen and they experience new side effects from TMS.
As mentioned above, our research included the analysis of a poll conducted by Hall in his Facebook group “Victims of TMS Action Group (VTAG)”. See visual below.24

In addition to the abovementioned Facebook community, VTAG is also running a dedicated TMS Side Effects website https://tms-sideeffects.com/, that aims “to improve the quality of life [our] members and anyone suffering side effects from TMS by sharing information, experiences and collaborating on solutions to all the respective legal, medical, and health issues that arise from the treatment.”
The website contains a written list of all side effects documented by James Hall via the VTAG Facebook community. Our team at TrueMedical contrasted the list from the website with the list obtained from our analysis of 20 TMS providers’ websites. As can be seen from the visualization below, the list of side effects from VTAG’s website is much more comprehensive.
What Are TMS Providers Not Telling Us?
The visual below compares the lists of side effects as provided by 20 TMS health providers’ websites we analyzed, with the list found on the official TMS Side Effects website. The two lists have only 3 side effects in common: seizures, headaches, and fatigue.

Does TMS Replace Antidepressants Completely?
TMS is touted as an effective alternative for depression treatment for patients dubbed treatment-resistant, meaning they do not respond to antidepressants as the first line of treatment.25
When asked to comment on whether patients who undergo TMS continue to take antidepressants, Hall said that many attempt to use TMS as a part of a plan to reduce or discontinue antidepressant use. “Still, it appears that the vast majority of people who undergo TMS are continuing to take psychiatric medications including antidepressants.”
How Do the Side Effects of TMS Compare to Those of Antidepressants?
Most TMS treatment providers and TMS device manufacturers claim that TMS does not come with the risk of side effects caused by antidepressants.8-19,26-32 This is one of their key talking points. According to them, this is a quintessential benefit of this form of therapy which is ideally suited for patients diagnosed with major depressive disorder who have failed to respond to medications adequately.
For instance, Johns Hopkins insists that TMS does not have the side effects associated with the use of antidepressant medications, citing sedation, sexual dysfunction, weight gain, and gastrointestinal upset.4 But conveniently enough, these are just some items on what is a very long list of side effects associated with different antidepressants.25
According to the reports of patients with hands-on experience, the potential side effects of TMS do not differ from the possible side effects of antidepressants.24
What Do Manufacturers Claim About TMS Side Effects?






The problem with these claims by manufacturers is that the evidence is still inconclusive because there are a lot of unknowns yet to be explored.22
For instance, the claim that TMS does not affect cognitive function has been proven inaccurate. This makes it alarming even if it does not raise particular safety issues and may even benefit the patient.22
According to a research on the effects of TMS “in the cognitive domain, TMS can produce desired (usually within the frame of the experimental design) and undesired, potentially long lasting, changes”.22
According to the Agency for Healthcare Research and Quality, there is not enough research to say how likely people are to experience side effects because of TMS therapy.33
We asked Hall if the name of a certain TMS device manufacturer has come up more often than others when it comes to patient complaints:
Neurostar has by far the most complaints, however Neurostar has the largest market share of TMS devices in use in the US market. Patients have complained of serious long-lasting, life-altering harms across all TMS devices. All TMS device manufacturers unilaterally deny claims of harms to patients directly, publicly, and to the FDA.
What Are the Key Issues with TMS in the U.S.?
Based on our in-depth analysis, we can list a few key issues with how TMS is marketed and used in the U.S.:
- Not enough is known about the variables that may impact response to TMS or the potential risks involved.<sup>22</sup>
- There is no guarantee that the therapy will be effective for every patient, and it may even make the situation worse for them.<sup>22</sup>
- The risks may outweigh the benefits, especially in the case of patients battling depression with psychosis, bipolar disorder or a high risk of suicide.<sup>34</sup>
- TMS is not non-invasive.<sup>7</sup>
- Patients are not given a transparent forewarning of the potential side effects and repercussions of this form of brain stimulation because the clinics which offer TMS in the U.S. withhold vital information, as do TMS device manufacturers.<sup>8-19,26-32</sup>
- Long-term side effects remain unknown.<sup>35</sup> Providers may also be reluctant to inform patients about the risk of potential unwanted or unintended long-term effects of stimulation, which can greatly <strong>“outlast the stimulation phase, sometimes up to several weeks after the end of a stimulation session”</strong>.
The only conclusion we can draw is this: clinics deliberately highlight the benefits of TMS while not only downplaying the potential side effects but actually keeping patients deliberately in the dark as to the scope and extent of the potential side effects.
Although the information is out there, you have to dig deep and reach out to patients with hands-on experience to learn about the possible side effects.
The bottom line is that it is currently impossible to determine with certainty the scope and intensity of side effects TMS can cause. The providers are not the only culprits for failure to disclose information: the manufacturers may share some of the responsibility. We are yet to see how this will play out and what the future has in store for patients battling depression, and whether we can expect safer alternatives to TMS and other forms of brain stimulation therapy for the treatment of depression in the years to come.
Methodology:
1. To choose the websites/web pages to analyze, we turned to the list of America’s Best Hospitals for 2021-22 compiled by the U.S. News https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview. With the domain rating of 91 (source: Ahrefs) and ranking #554 on Alexa Rank, the U.S. News satisfied our criteria for a credible source of most popular hospitals in the USA. We then searched for a web page on each official website that deals with TMS. For some hospitals, there were two dedicated TMS pages, which we also included in our analysis (UCLA Medical Center and Johns Hopkins Medicine). This is why we refer to our units of analysis as “web pages” as well as “websites”, for clarity. We identified the following aspects to analyse the text against:
- How many side effects were mentioned
- Which side effects were mentioned
- Stated there were other side effects but didn’t specify, or other notes
- Explicitly refers to TMS as non-invasive
- Clearly states that the treatment may not be effective for everyone
- Clearly states that some groups shouldn’t receive treatment
- States the duration of side effects
- Use of vague language (including passive voice, modal verbs) or logical fallacies (for example, exaggeration)
- Vague language or logical fallacy examples
- Links to third-party studies
- Patient testimonials
- Discrepancies
The analysis was both quantitative and qualitative. The results of the quantitative analysis has been presented in this article in the form of figures, whereas the results of the qualitative analysis has been presented in the form of Discussion throughout the article, as well as direct quotes from the web pages, serving as illustrations.
2. We decided to include James Hall in the article due to his prominent role in raising the awareness of all the potential side effects of TMS. As the founder of the Facebook group “Victims of TMS Action Group (VTAG)”, he has collected patients’ stories and served as a spokesperson. We conducted an email interview with Mr Hall, and used his responses throughout the article as paraphrases and direct quotes. The article has been revised and approved by Mr Hall. We also extensively used the information from https://tms-sideeffects.com/, the official website of the VTAG group.
Sources
- Harvard Health Publishing. (2018). Transcranial magnetic stimulation (TMS): Hope for stubborn depression.
- American Psychiatry Association. (2019). What is Electroconvulsive therapy (ECT)?.
- Biju Basil, MD, DPM, Jamal Mahmud, MD, DPM, Maju Mathews, MD, MRCPsych, Dip PSYCH,corresponding author Carlos Rodriguez, MD, and Babatunde Adetunji, MD, DPM. (2005). Is There Evidence for Effectiveness of Transcranial Magnetic Stimulation in the Treatment of Psychiatric Disorders?
- Johns Hopkins Medicine. (2021). Frequently Asked Questions About TMS.
- UNC School of Medicine. (2021). TMS FAQ.
- TMS and Brain Health. (2020). What is the success rate of TMS Therapy?
- Nick J. Davis and Martijn G. van Koningsbruggen. (2013). “Non-invasive” brain stimulation is not non-invasive.
- Mayo Clinic. Mayo Clinic.
- Johns Hopkins Medicine. Johns Hopkins Medicine.
- McLean Hospital. McLean Hospital.
- Massachusetts General Hospital. Massachusetts General Hospital.
- UCSF Medical Center. UCSF Medical Center.
- New York-Presbyterian Hospital-Columbia and Cornell. New York-Presbyterian Hospital-Columbia and Cornell.
- Rush University Medical Center. Rush University Medical Center.
- University of Michigan Hospitals-Michigan Medicine. University of Michigan Hospitals-Michigan Medicine.
- Stanford Health Care-Stanford Hospital. Stanford Health Care-Stanford Hospital.
- Hospitals of the University of Pennsylvania-Penn Presbyterian. Hospitals of the University of Pennsylvania-Penn Presbyterian.
- Brigham and Women’s Hospital. Brigham and Women’s Hospital.
- Houston Methodist Hospital. Houston Methodist Hospital.
- Florida TMS Clinic. (2021). TMS Side Effects [Read Before Doing TMS].
- TMS-SideEffects. (2021). TMS Side Effects.
- Simone Rossi, Mark Hallett, Paolo M. Rossini, Alvaro Pascual-Leone, and The Safety of TMS Consensus Group. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research.
- U.S. Food & Drug Administration. (2017). MAUDE Adverse Event Report: NEURONETICS NEUROSTAR TMS NEUROSTAR TMS.
- Facebook. Victims of TMS Action Group (VTAG).
- Cleveland Clinic. (2019). Depression Medicines.
- Neurostar. Side Effects.
- MagVenture. Are you not responding to antidepressants?
- BrainsWay. Are there any safety concerns or side effects related to Deep TMS therapy?
- My CloudTMS. Are there any side effects with TMS therapy?
- Apollo. Side Effects and Safety.
- Nexstim. Depression: Risks and Side Effects.
- MagStim. Patient FAQs.
- Agency for Healthcare Research and Quality. 2012. Therapies for Treatment Resistant Depression: A Review of the Research.
- National Alliance on Mental Illness (NAMI): ECT, TMS And Other Brain Stimulation Therapies.
- National Institute of Mental Health: Brain Stimulation Therapies.
- PubMed: Anxiety induced by repetitive transcranial magnetic stimulation is suppressed by chronic treatment of paroxetine in rats
